FARCO-PHARMA looks back on more than six decades of company history. It all started with a ground-breaking idea and cooperation. What followed is a great success story. And it continues! Thanks to a global network of more than 45 distribution partners, FARCO-PHARMA products are now available worldwide.
The founder: Prof. Dr. med. Carl Erich Alken
Prof. Dr. Alken lays the foundation for success by developing the idea of instilling lubricant directly into the urethra during prostate screening examinations. This method reduced the risk of injury and led to the invention of the first sterile lubricant in a pre-filled syringe.

Foundation of FARCO-PHARMA
FARCO-PHARMA GmbH is founded through the merger of FARCO AS from Copenhagen and the pharmacist Karl-Heinz Reinhard from Launsbach.
FARCO-Gel: First lubricating gel in a syringe worldwide
Relocation of the company headquarters to Cologne
Due to increasing demand, filling in pharmacies can no longer be guaranteed. The company headquarters of FARCO-PHARMA are relocated to Cologne following the takeover of Klosterfrau, where FARCO-PHARMA has been based ever since.
Optimization of Instillagel®
Expansion in Europe
Production in Berlin

Introduction of Endosgel®
Expansion in Great Britain
Expansion into Asia
Focus on sterile pre-filled syringes
Introduction of steam sterilization
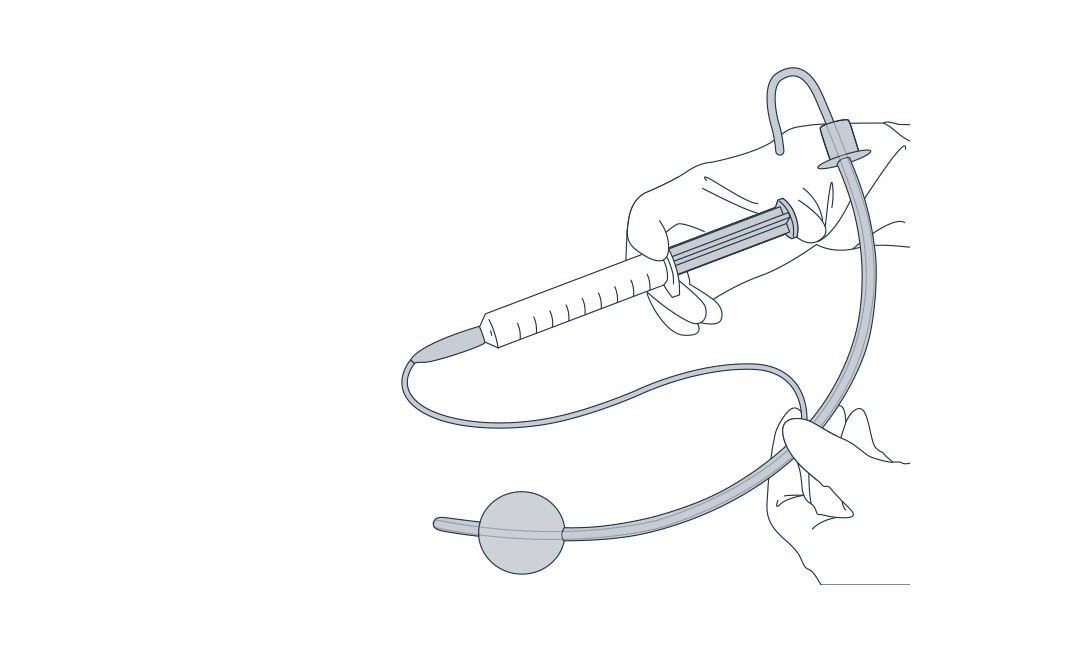
New division: solution for catheter inflation

Introduction of InstillaGel® Lubri
Expansion into North America
Expansion to Australia
Expansion into South America
Expansion into the USA
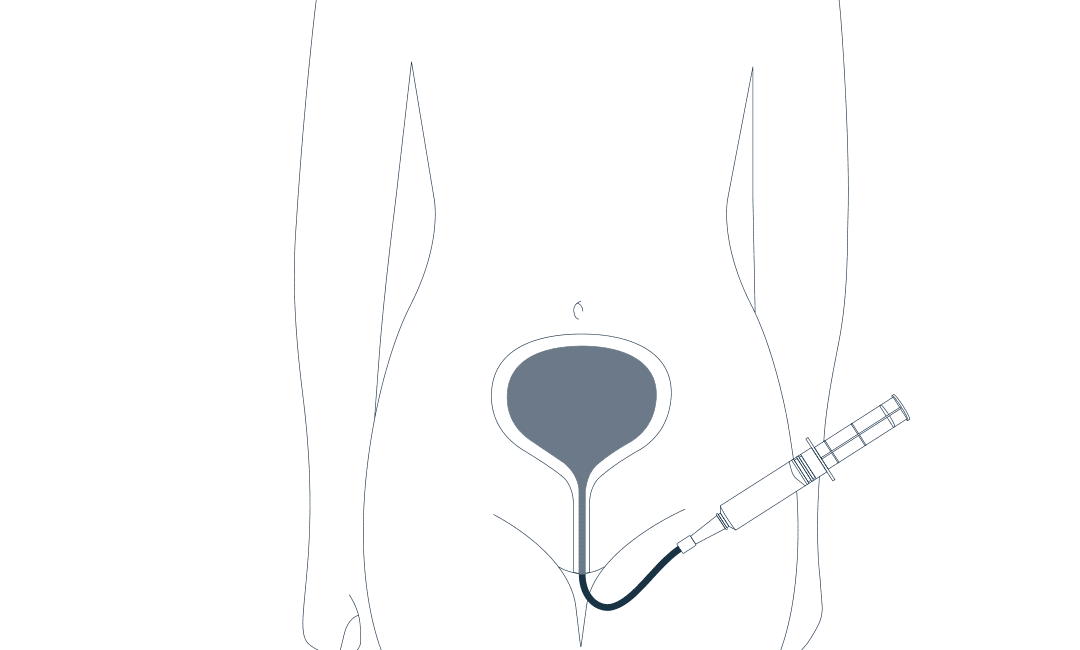
Introduction of instillation therapies for the urinary bladder
FARCO launches the first therapy option for the treatment of chronic urinary bladder diseases.
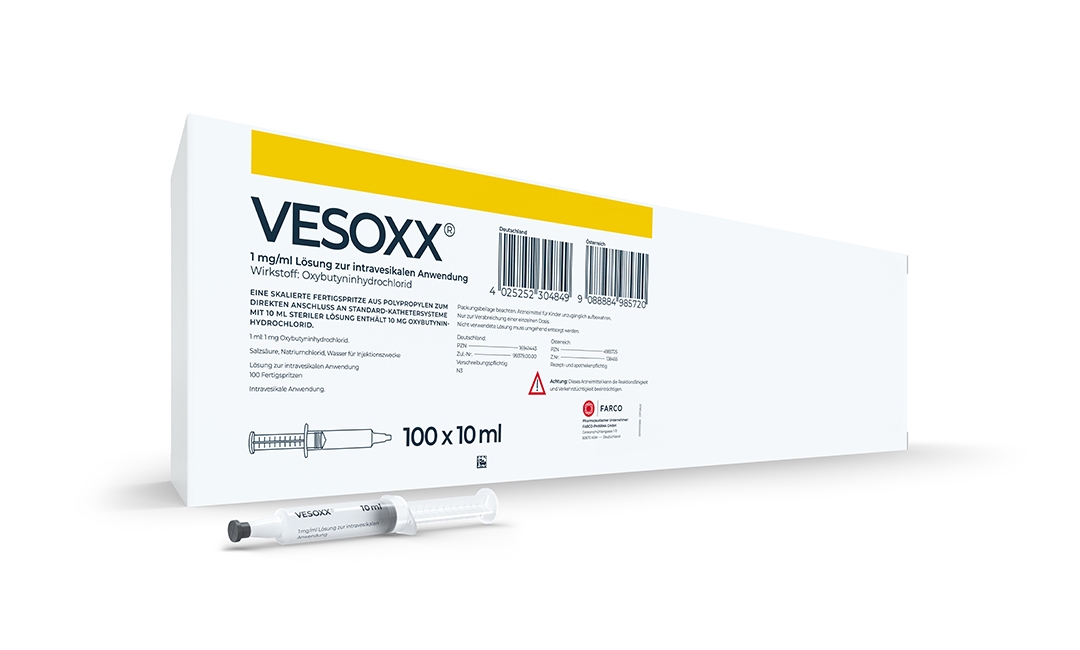
VESOXX®: First approved instillation therapy with oxybutynin worldwide

Distribution rights for Deflux® in the DACH region
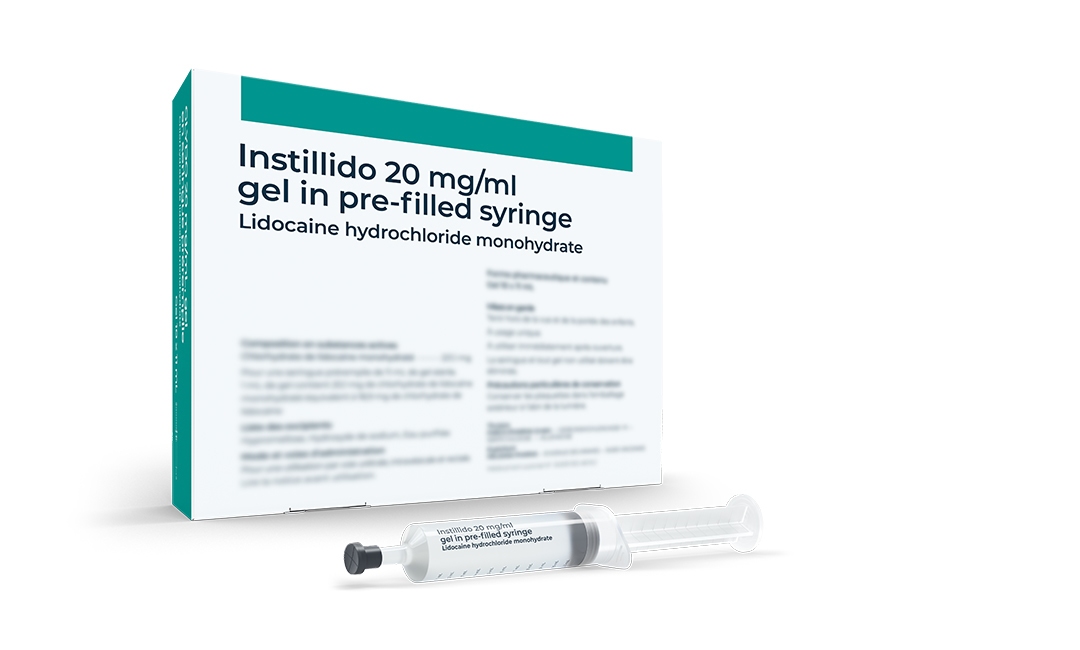
Introduction of Instillido®
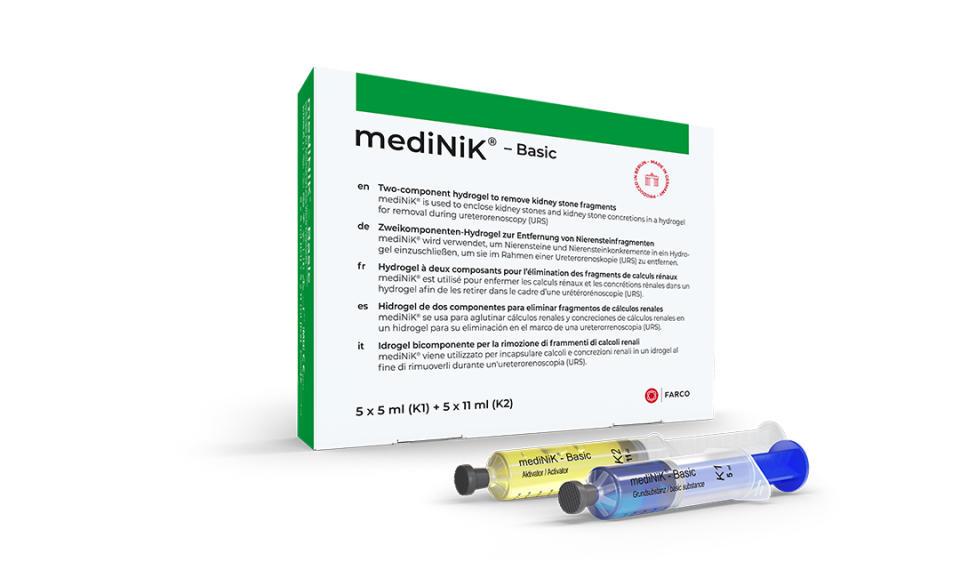
Innovative kidney stone removal with mediNiK®

Strong partner in urology

1958
The founder: Prof. Dr. med. Carl Erich Alken
Prof. Dr. Alken lays the foundation for success by developing the idea of instilling lubricant directly into the urethra during prostate screening examinations. This method reduced the risk of injury and led to the invention of the first sterile lubricant in a pre-filled syringe.
Foundation of FARCO-PHARMA
FARCO-PHARMA GmbH is founded through the merger of FARCO AS from Copenhagen and the pharmacist Karl-Heinz Reinhard from Launsbach.

1964
1967
FARCO-Gel: First lubricating gel in a syringe worldwide
FARCO-PHARMA launches the world’s first lubricating gel in a syringe, which is registered as the Instillagel® brand.
Relocation of the company headquarters to Cologne
Due to increasing demand, filling in pharmacies can no longer be guaranteed. The company headquarters of FARCO-PHARMA are relocated to Cologne following the takeover of Klosterfrau, where FARCO-PHARMA has been based ever since.
1970

1971
Optimization of Instillagel®
Instillagel® is further developed into a stable and sterile lubricant gel and manufactured at the new production site in Cologne. This makes Instillagel® the first sterile lubricant in a pre-filled syringe. Instillagel® is also exported to Austria for the first time.
Expansion in Europe
Instillagel® is distributed in Switzerland, the Netherlands, Belgium and Luxembourg.
1970’s

1976
Production in Berlin
Production is relocated to Berlin to expand production capacity through the use of robot technology. All FARCO products are still produced there today.
Introduction of Endosgel®
Endosgel®, a sterile lubricating gel with disinfectant properties, is launched.

1978
1987
Expansion in Great Britain
Instillagel® is launched in England and quickly establishes itself as the market leader.
Expansion into Asia
Hong Kong is the first Asian export country to be supplied.

1990

1995
Focus on sterile pre-filled syringes
From 1995, FARCO concentrates on pharmaceuticals and medical products in sterile pre-filled syringes for urology and related specialties.
Introduction of steam sterilization
For the first time, FARCO is using steam sterilization to ensure effective germ destruction and preserve the essential characteristics of the product.Steam sterilization is considered the gold standard for the sterilization of pharmaceuticals and medical devices.

1996

1997
New division: solution for catheter inflation
With Farco-fill® Aqua-Glycerol, the portfolio has been expanded to include a solution for catheter inflation.
Introduction of InstillaGel® Lubri
An active ingredient-free lubricant gel for patients with intolerances is introduced.

2001
2006
Expansion into North America
Canada is the first country in North America to be supplied.
Expansion to Australia
FARCO products are distributed in Australia.
2008
2012
Expansion into South America
Colombia is the first South American country to be supplied.
Expansion into the USA
FARCO receives FDA approval for the sterile lubricant GLYDO® in the USA.
2014

2017
Introduction of instillation therapies for the urinary bladder
FARCO launches the first therapy option for the treatment of chronic urinary bladder diseases.
VESOXX®: First approved instillation therapy with oxybutynin worldwide
VESOXX® is launched as the first intravesical oxybutynin therapy in Germany.

2020

2021
Distribution rights for Deflux® in the DACH region
FARCO acquired the distribution rights for Deflux®, a product for the treatment of vesicoureteral reflux.
Introduction of Instillido®
A sterile lubricating gel with a local anaesthetic effect is launched as a medicinal product.

2022

2023
Innovative kidney stone removal with mediNiK®
With the acquisition of Purenum GmbH, FARCO expands its portfolio to include the mediNiK® hydrogel method for the removal of kidney stone fragments.
Strong partner in urology



